[ Instrument R & D of Instrument Network ] Recently, the team of the Extreme Environment Quantum Matter Center of the Institute of Solid Physics of the Hefei Academy of Material Sciences, Chinese Academy of Sciences used a diamond to press the anvil pressure device to study the physical properties of sulfur dioxide under high pressure. For the first time, pressure-induced Reversible amorphous polymorphic phase transition, related research results were published in the Proceedings of the National Academy of Sciences (PNAS) under the title Pressure-induced amorphization and existence of molecular and polymeric amorphous forms in dense SO2.
Sulfur dioxide is a simple molecular gas that plays an important role in industrial production, geophysics, and the atmospheric environment. Unlike common gases such as nitrogen, oxygen, and carbon dioxide, sulfur dioxide is a curved polar molecule that exhibits different properties and phase diagrams under high pressure. In this work, researchers found that pressure induces amorphization of sulfur dioxide, and there are two high-pressure amorphous phases: molecular amorphous phase (two-coordinated sulfur) and polymer amorphous phase (three-coordinated sulfur). This is the first amorphous polymorphism found in a sulfur dioxide system.
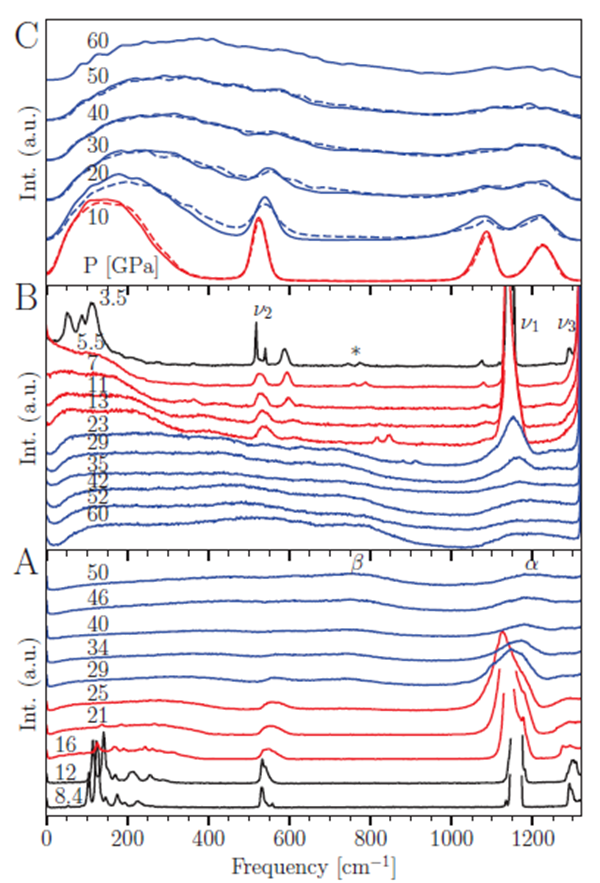
Researchers of the Quantum Matter Center at the Institute of Extreme Environment of the Institute of Solid Materials used a high-pressure device such as a diamond anvil to pressurize sulfur dioxide to 60 GPa, combined with low-temperature means, and using Raman spectroscopy, synchrotron radiation X-ray diffraction and other technologies, a pressure range of 0-60 GPa Phase change and structural information in the 77-300K temperature range. At a temperature of 77K, when the applied pressure is lower than 16GPa, sulfur dioxide is in the crystalline phase. After being pressurized to 16GPa, sulfur dioxide starts a pressure-induced amorphous phase transformation, and enters the molecular amorphous phase (bi-coordinated sulfur) from the crystal phase. Further pressurized to 26GPa, sulfur dioxide has undergone a phase transition from a molecular amorphous phase (two-coordinated sulfur) to a chain polymerized amorphous phase (tri-coordinated sulfur). Through experiments with different temperature paths, it was found that sulfur dioxide follows the phase transition path of crystalline phase-molecular amorphous phase-polymer amorphous phase in the entire temperature range of 77-300K, and the phase transition path is reversible, and further research found that different temperature paths The pressure of the amorphized phase transition under the pressure is different, but it is maintained between 10-16GPa. The researchers also verified the phase transition path through molecular dynamics simulations, and found that the phase transition from the amorphous phase of the molecule to the amorphous phase of the polymer is essentially that the S atom in the sulfur dioxide molecule changes from two coordination to three coordination, thus A chain polymer was formed. The researchers through the perfect combination of experimental and theoretical means, confirmed the existence of amorphous polymorphism in the sulfur dioxide system.
Under high pressure, structural phase transitions between crystalline phases of substances are relatively common, and phase transitions between amorphous phases are very rare. Some materials have multiple amorphous phases, and the conversion between different amorphous phases is called amorphous polymorphic phase transition. Amorphous polymorphism was discovered in the water / ice system in 1984. It is a low-density amorphous ice (LDA) and a high-density amorphous ice (HDA), followed by silica, silicon and germanium dioxide. Amorphous polymorphism is also found in. In condensed matter physics, the phenomenon of amorphous polymorphism is very interesting, but there are few systems to find this phenomenon, and the research is not systematically in-depth. This work not only provides a new example for amorphous polymorphism, but also provides a reference for understanding the amorphous phase transition of other materials.
This work was supported by the National Natural Science Foundation of China, the International Visiting Scholars Program of the Chinese Academy of Sciences, the Innovation Fund of the Chinese Academy of Sciences, and the Dean ’s Fund.
Embossed Silicone Bracelets
Embossed silicone bracelet introduction:
There are millions of silicone bracelets in the market,however,the embossed silicone bracelet is always in the majority ,the raised 3d feature of colorful logos and figures catch more people's eye,and it's embossed feeling win more customers'favor!
Embossed silicone bracelet description:
1.Product name:Embossed silicone bracelets,embossed bracelet,custom silicone bracelets,custom mens bracelet,custom name bracelets,custom silicone wristbands
2.Place of origin:Guangdong China
3.Color:any pantone color is available
4.Logo:3d/embossed
5.MOQ:500pcs.
6.Package:1 pcs/opp,customized design is available.
7.Design:Customized
8.Certification:FDA,LFGB,SGS,ROHS,etc.
9.Usage:Gifts/Sports
10.Embossed silicone bracelet photos for reference