Top-grade NMN Raw Material Powder,NMN Raw Material Powder for Longevity,Reliable NMN Raw Material Shanghai CANAL Material Technology Co., Ltd , https://www.nmncanal.com![]()
Spin compound-regulated charge recombination path and dynamics revealed by Dalian Chemical Institute
[ Instrument Network Instrument R & D ] Recently, Wu Kaifeng, a researcher in the Research Group of Optoelectronic Materials Dynamics Special Zone of the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, used femtosecond transient spectroscopy to reveal inorganic / organic hybrids based on semiconductor quantum dots and organic acceptor molecules. There are spin-regulated charge recombination paths and dynamics in the system.
Hybrid systems of inorganic semiconductor quantum dots (QD) and organic molecules have important application prospects in the fields of photocatalysis, light-emitting devices, and triplet sensitization. A micro-process critical to these applications is the charge transfer and recombination dynamics at the QD / molecular interface. To this end, researchers have conducted extensive research on these dynamic processes, revealing the basic principles of regulating charge transfer and compound dynamics through factors such as energy driving forces and electron coupling strength. However, the use of electron spin as a new dimension to regulate charge transfer and recombination dynamics has been rarely reported.
Quantum dots (quantum dots) are semiconductor nanostructures that bind excitons in three spatial directions. Sometimes called "artificial atoms", "superlattices", "superatoms" or "quantum dot atoms", it is a new concept introduced in the 1990s. This constraint can be attributed to the electrostatic potential (generated by external electrodes, doping, strain, and impurities), the interface between two different semiconductor materials (for example, in self-assembled quantum dots), and the surface of a semiconductor (for example: semiconductor nanocrystals) ), Or a combination of the three. Quantum dots have a separate quantized energy spectrum. The corresponding wave function is located in the quantum dot in space, but extends over several lattice periods. A quantum dot has a small number (1-100) of an integer number of electrons, holes, or electron hole pairs, that is, the amount of electricity it carries is an integer multiple of the elementary charge.
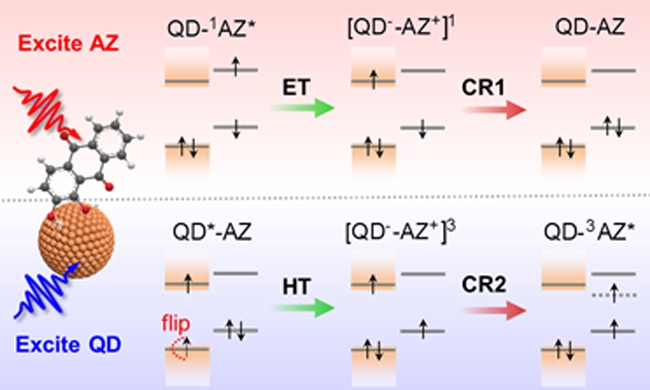
To explore the possibility of spin regulation, the research team designed a QD-AZ hybrid material based on CdS QD and Alizarin molecules (AZ). The spectral characteristics of the system allow the selective choice of excited QD or AZ, respectively, which can trigger charge separation.
Charge separation refers to the fact that in the entire plasma, usually due to the different properties of electrons and positive ions (with different masses, charges and pressures, etc.), under the action of electric, magnetic or gravitational fields, speeds of different magnitudes and directions are obtained. The charge density distribution of electrons and positive ions is also different, and space charges appear in the plasma. This phenomenon is called charge separation.
Specifically, excitation of AZ causes electrons to be injected into the conduction band of QD, forming a QD--AZ + charge separation state. The charge-separated state finally recombines back to the ground state, which is consistent with the observations made in the team's previous work (J. Am. Chem. Soc. 2018). Selective excitation of QD can also trigger the transfer of holes to AZ to form the QD-AZ + charge separation state. However, the recombination of this separation state does not return to the ground state, but instead produces a very long-lived species, which seems to be counterintuitive. The research team has recently accumulated a certain working basis in the field of triplet energy transfer in QD-molecular systems (Nat. Commun. 2020; J. Am. Chem. Soc. 2019; J. Am. Chem. Soc. 2019). Inspired by this, the species is speculated to be the spin triplet (3AZ *) of the AZ molecule, and verified by triplet sensitization experiments.
The mechanism analysis shows that the difference in charge recombination paths caused by this selective excitation is essentially regulated by the spin flipping dynamics. The QD--AZ + charge-separated state produced by exciting AZ is a spin singlet state, and because the electron spins in the II-VI QD are slower to reverse (weak spin-orbit coupling), the charge-separated state can maintain a singlet state for a long time. Characteristics until the compound returns to the ground state. On the contrary, in the case of excited QD to trigger hole transfer, due to the extremely fast spin rotation (strong spin-orbit coupling) of the hole spins in the II-VI QD, the QD--AZ + charge separation state generated is actually a spin triplet state leading. The recombination of the charge-separated states preferentially generates the spin triplet state of the AZ molecule.
Electron spin is one of the basic properties of electrons. Abbreviation for quantum number of electron intrinsic motion or electron intrinsic motion. In 1925, GE Uhlenbeck and SA Guzmit were inspired by Pauli's incompatibility principle, analyzed some experimental results of atomic spectrum, and proposed that electrons have intrinsic motion—spin, and that they are related to electron spin. Spin magnetic moment. This can explain the fine structure of the atomic spectrum and the anomalous Zeeman effect. The spin angular momentum of the electron is shown in the figure, where the electron spin S = 1/2. In 1928, PAM Dirac proposed the electron's relativistic wave equation. The equation naturally includes the electron spin and the spin magnetic moment. Electron spin is a quantum effect and cannot be understood classically. If electron spin is viewed as a rotation around an axis, a contradictory result is obtained.
The use of electronic spins to control charge transfer and compound paths has important guiding significance for the application of these hybrid materials in light-driven energy conversion.
Related research results were published in the Journal of the American Chemical Society (J. Am. Chem. Soc.). The above work was supported by the National Natural Science Foundation of China, the National Key R & D Program, the Clean Energy Pilot Project of the Chinese Academy of Sciences "Essence and Regulation of Energy Chemical Conversion", and the Liaoning Province Xingliao Talent Program.
Source: Dalian Institute of Chemical Physics, Encyclopedia